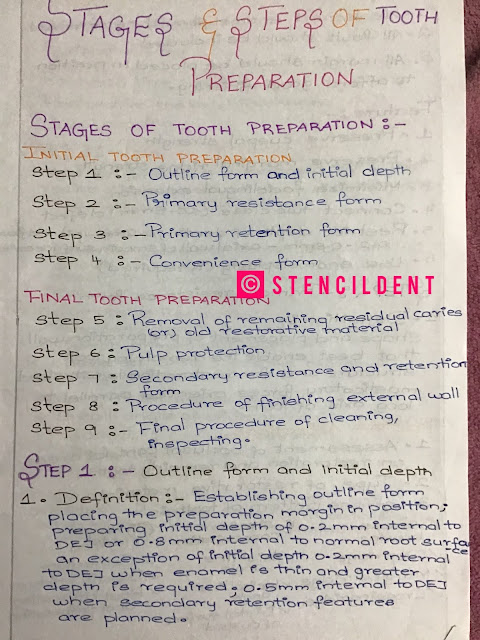
Tarnish and Corrosion
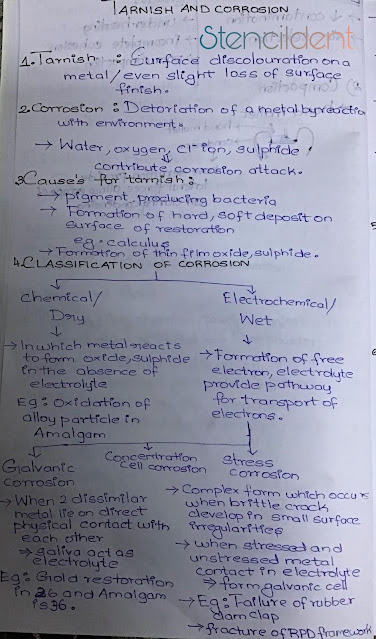
TARNISH AND CORROSION
TARNISH : Surface discoloration on a metal /even slight loss of surface finish.
CORROSION: Deterioration of a metal by reacting with environment,water ,oxygen,chloride ion,sulfide contribute corrosion attack.
CAUSES FOR TARNISH :
- Pigment producing bacteria
- formation of hard ,soft deposit on surface of restoration (eg-calculus)
- formation of thin film oxide and sulphide.
CLASSIFICATION OF CORROSION
- CHEMICAL/DRY :
- In chemical type of corrosion metal reacts to form oxide,sulphide in the absence of electrolyte
- for an example : oxidation of alloy particle in amalgam.
- In electrochemical type of corrosion there will be formation of free electron ,electrolyte that provide pathway for transport of electrons.
- types of electrochemical corrosion : A) galvanic corrosion B) concentration cell corrosion and C) stress corrosion
A)Galvanic corrosion:
- When two dissimilar metal lie on direct contact with each other ,saliva acts as a electrolyte that provides pathway for transport of electrons .
- For example: If there is gold restoration in 26 and amalgam in 36 that is if there is dissimilar metal restoration in opposing tooth that comes in contact galvanism occurs.
- Amalgam forms an anode and start corroding .
- It produces sharp pain called galvanic shock.
- This galvanic that's produced can be minimised by painting varnish on amalgam restoration,therefore its advised to avoid dissimilar metal.
- Galvanic shock can also arise in a single metal restoration when a metallic surface is brought close to the tooth such as focke.
- concentration cell corrosion occurs when a liquid corrosive is entrapped in gaps between metals.
- i)Electrolyte concentration cell: in metallic restoration ,food debris partially cover,concentration of electrolyte differ from saliva because of corrosion.
- ii)Oxygen concentration cell: corrosion occurs on restoration having lower concentration of oxygen.
C) Stress corrosion:complex form which occurs when brittle crack develop in small surface irregularities,when stressed and unstressed metal contact in electrolyte it forms a galvanic cell
- example- failure of rubber dam clap,fracture of rpd framework.
FACTORS AFFECTING CORROSION OF RESTORATION
1) Diet
2)Drug
3)Smoking
4)Bacterial activity
5)Oral hygiene,habits.
PROTECTION AGAINST CORROSION
1)PASSIVATION:
oxide layer forms on surface of metal that protect from corrosion
example-titanium,chromium
2)INCREASING NOBLE METAL CONTENT:
The more positive electromagnetic force indicates its more corrosion and tarnish resistant.
3)POLISHING:
polishing the metallic restoration to high lusture can act as a protection against corrosion.for example- amalgam,cast metal.
4)OTHER:
Dissimilar metal on opposing arch must be avoided in order to prevent galvanic corrosion.
THANK YOU



Comments
Post a Comment